Phase 3 Drug Trial Shows Lung Function Improvement for Patients with aPAP
Research By: Bruce Trapnell, MS, MD
Post Date: August 22, 2025 | Publish Date: August 20, 2025
Study in NEJM, led by Bruce Trapnell, MD, evaluated the drug molgramostim
A drug called molgramostim resulted in significant improvement in lung function and lung health-related quality of life in people with autoimmune pulmonary alveolar proteinosis (aPAP), according to a study in The New England Journal of Medicine led by an expert at Cincinnati Children’s.
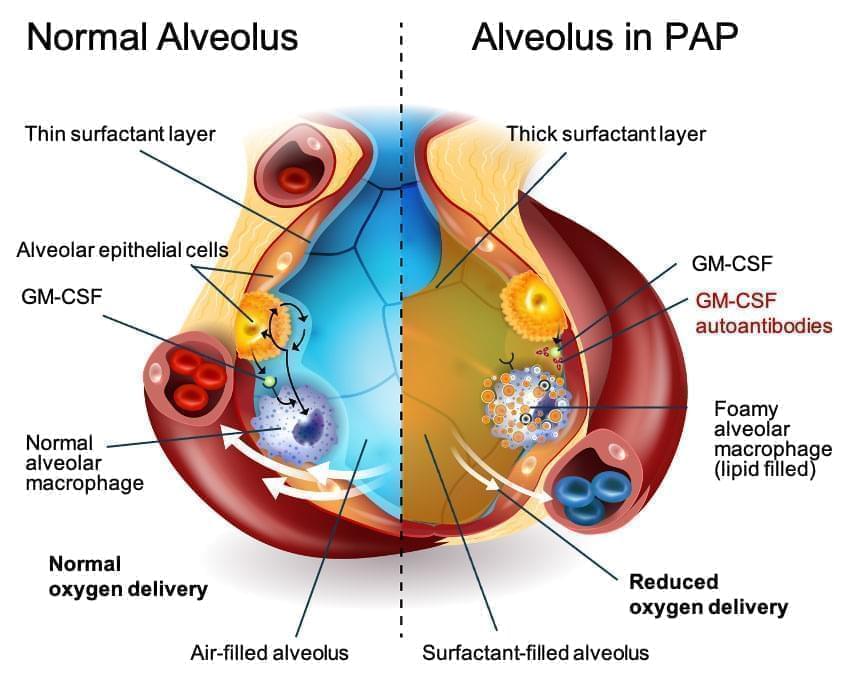
APAP is a rare lung disease, with an estimated 10 people in a million living with the condition. The disease causes an abnormal build-up of surfactant in alveoli, the lung’s air sacs. As the excess surfactant accumulates, it causes symptoms including shortness of breath, cough, and frequent fatigue. Long term, the disease can lead to lung fibrosis and the need for a lung transplant.
Currently, the primary treatment to manage aPAP is a procedure called whole-lung lavage, which physically washes out the excess surfactant sediment. However, experts have been working for years to develop an inhaled treatment to provide a version of the granulocyte-macrophage colony-stimulating factor (GM-CSF) that restores the ability of the lung cells to remove the excess surfactant.
Bruce Trapnell, MD, director of the Translational Pulmonary Science Center at Cincinnati Children’s, has studied this condition for decades. He served as lead author of the NEJM publication and as lead clinical investigator for the IMPALA-2 clinical trial.
The trial was conducted at 43 sites in 16 countries, including the U.S., Canada, Japan, France, Germany, Italy, Ireland, Romania, Denmark, South Korea, Australia, and Turkey.
“Treatment with molgramostim improved the cardinal manifestations of aPAP, namely it reduced pulmonary surfactant burden and improved pulmonary gas transfer, respiratory health-related quality of life, and patient functionality. Additionally, molgramostim was well tolerated with no notable safety concerns,” Trapnell said in a media release from the drug maker Savara.
Life Improving Results
After 48 weeks of daily doses, study participants receiving molgramostim saw an 11.6% improvement in pulmonary gas transfer compared to 4.7% improvement among those receiving a placebo.
Patients also showed improved exercise capacity on treadmill tests and reported improved quality of life as measured by the St. George’s Respiratory Questionnaire (SGRQ).
The treatment did not eliminate the need for whole-lung lavage but did appear to reduce the need. While 11 patients in the placebo group received at least one procedure during the study period, only six patients treated with molgramostim needed the procedure.
A Research Marathon, Nearly Complete
For Trapnell, the latest clinical study published in NEJM reflects the outcome of nearly 30 years of research.
Trapnell and colleagues figured out the pathogenesis of PAP in GM-CSF deficient mice, established that GM-CSF was required by human alveolar macrophages, and characterized aPAP as distinct from other types of PAP. The team also developed and reported a blood test in 2014 that serves as the global gold standard for diagnosing aPAP.
“More than 750 aPAP patients have been diagnosed with this assay,” says Brenna Carey, MS, PhD, a co-author on this study who leads the clinical research testing program in the Translational Pulmonary Science Center.
Trapnell then worked with the drug maker, Savara, to shepherd the treatment through the full series of human clinical trials. While Savara funded the final stages of clinical trial work, grants totaling $4.9 million over 19 years — primarily from the National Institutes of Health — sustained Trapnell’s work. The research was supported by Republican and Democrat administrations dating back to President Clinton.
“This report documents the safety, tolerability, and clinical efficacy of inhaled molgramostim as therapy of aPAP,” he says. “I believe this will change clinical practice and support the regulatory approval of molgramostim as therapy of aPAP.”
What’s next?
Savara officials have stated they intend to seek FDA approval for the treatment. Meanwhile, Trapnell’s work continues.
“There are still questions to be answered, including those related to dosing and administration and the time required to achieve maximal therapeutic benefit,” he says. “We will continue to pursue these questions in our efforts to improve the lives of people living with aPAP.”
Don’t Miss a Post:
- Subscribe to the Research Horizons Newsletter
- Follow Cincinnati Children’s Research Foundation on Bluesky, X and LinkedIn
| Original title: | Phase 3 Trial of Inhaled Molgramostim in Autoimmune Pulmonary Alveolar Proteinosis |
| Published in: | The New England Journal of Medicine |
| Publish date: | August 20, 2025 |
Research By