Organoid Technology Helps Reveal Possible Cause of Rare, Fatal Kidney Disorder
Research By: Raphael Kopan, PhD
Post Date: December 23, 2023 | Publish Date: Dec. 9, 2023
Autosomal recessive renal tubular dysgenesis (AR-RTD) is an extremely rare inherited disorder that results in missing or severely malformed proximal tubules that the kidney uses to remove wastes and return needed substances to the bloodstream. Nearly all those affected die in utero or within the first few days after birth.
Now, a study published Dec. 9, 2023, in Nature Communications, reports that a developmental delay in kidney vascularization basically starves growing tubules during a crucial point in their development, which appears to drive the disease.
The study was led by first author Naomi Pode-Shakked, MD, PhD, a former research fellow at Cincinnati Children’s now with the Tel-Aviv Sourasky Medical Center in Israel, and corresponding author Raphael (Rafi) Kopan, PhD, Division of Developmental Biology. Cincinnati Children’s co-authors included Megan Slack, BSc, research assistant Nambirajan Sundaram, PhD, Kyle McCracken, MD, PhD, and Michael Helmrath, MD.
Previous research has identified mutations in four genes that underlie the fatal disease. The new study leverages mutations in two of those genes: angiotensin converting enzyme (ACE) which cannot produce a signal (angiotensin ΙΙ or AngII), and the AngII receptor type 1 (AGTR1), which cannot “hear” the signal.
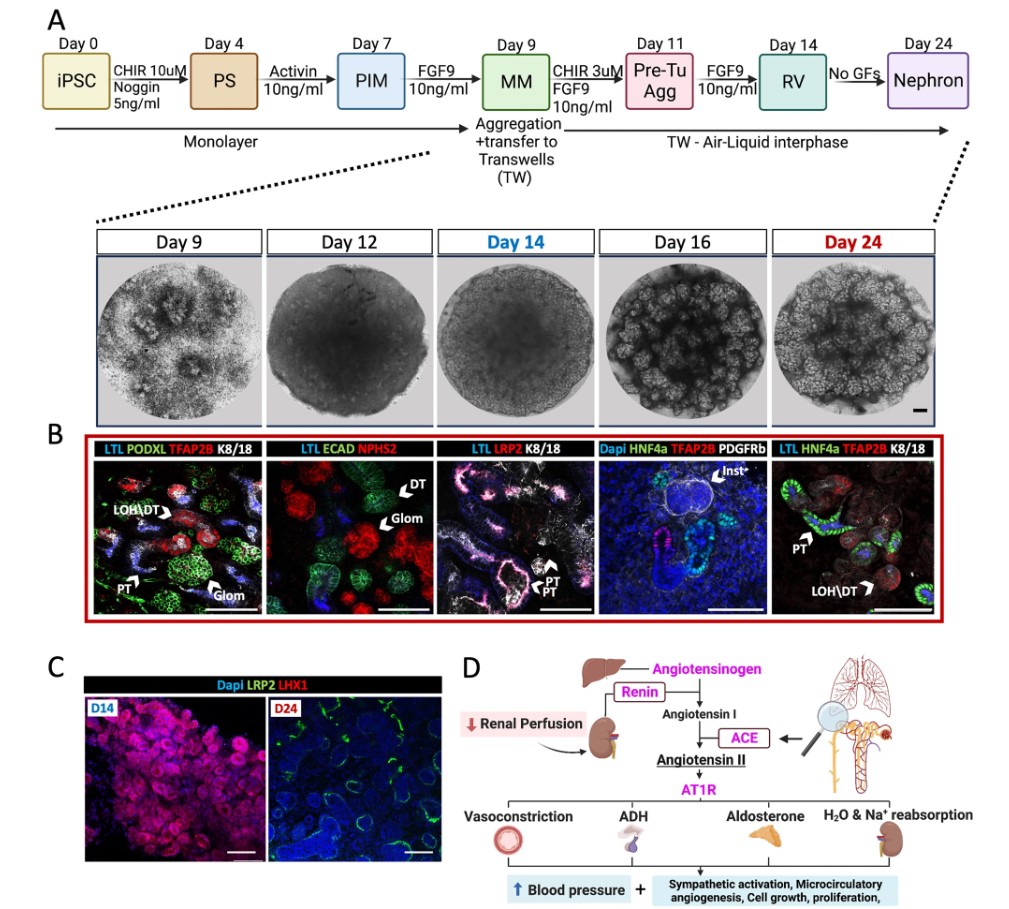
In a series of experiments, the research team tracked how these mutations affected the development of lab-grown kidney organoids derived from patients with AR-RTD and others engineered to harbor ACE or AGTR1 mutations. These were compared to organoids derived from the same donor but in which the mutations were either repaired or absent. All organoids fared well in a tissue culture incubator, in either normal or low oxygen conditions.
Surprisingly, organoids that cannot “hear” the host AngII signal (those with a mutated form of the gene AGTR1) failed to thrive when implanted in mice.
Subsequently, the team discovered that organoids without AGTR1 delayed production of vascular endothelial growth factor A (VEGF-A), a key protein that supports healthy microvascular growth. This delay did not impact growth in the incubator but proved critical for transplantation success. The impact of this delay was detected only by studying the process in transplanted organoids.
Re-introducing VEGF-A to Agtr1 mutant cells was enough to rescue transplantation and proximal tubules production. The team suggested that a delay occurring during a small window of human fetal development results in disrupted proximal tubule growth and AR-RTD.
The most common AR-RTD mutations in human fail to produce AngII. So, could administering AngII during human pregnancy help prevent this deadly kidney malformation?
Not without untoward effects, the researchers say. Unless a special delivery strategy can be developed, such a treatment would likely trigger life-threatening preeclampsia. However, the research points out that nutrient deficiency, and not oxygen deprivation, underlies AR-RTD.
Looking forward, the researchers suggest that a more promising approach to prevent AR-RTD would be to identify the critical nutrients that specifically support proximal tubule growth, and provide them as a supplement.
| Original title: | RAAS-deficient organoids indicate delayed angiogenesis as a possible cause for autosomal recessive renal tubular dysgenesis |
| Published in: | Nature Communications |
| Publish date: | Dec. 9, 2023 |
Research By

My laboratory is interested in mechanisms of Notch signaling, organogenesis, and disease processes.