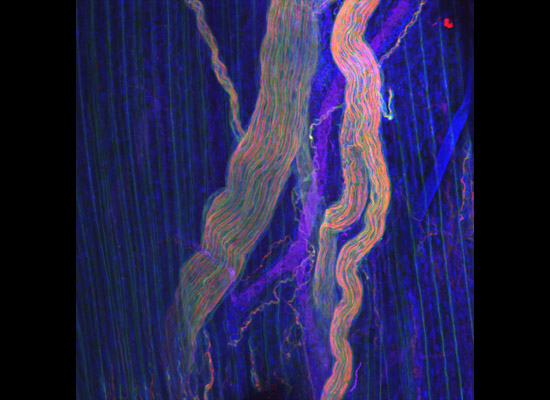
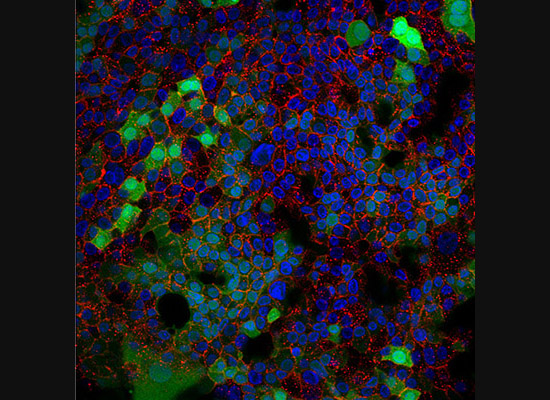
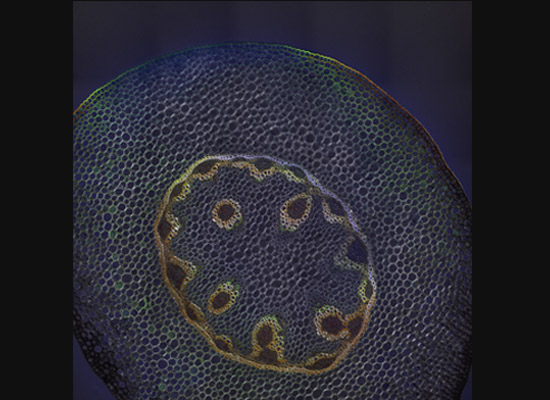
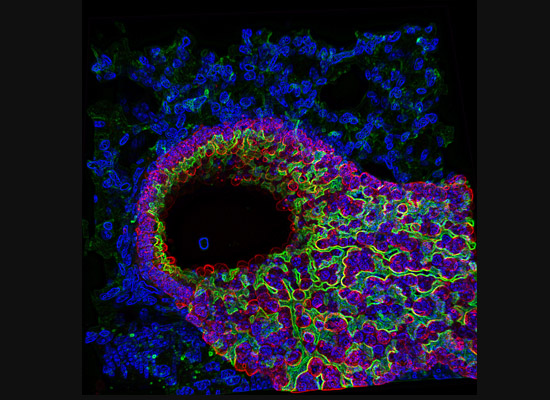
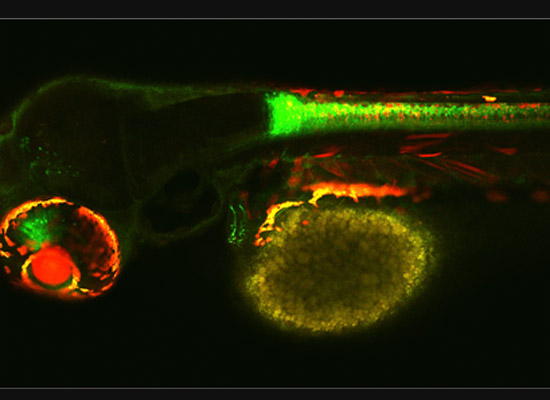
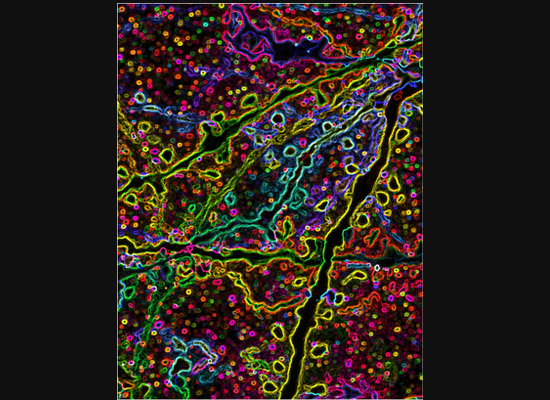
A gastroenterology researcher in the Confocal Imaging Core’s (CIC) analysis suite pores over a three-dimensional, color-splashed snapshot of organoids on a computer monitor. He rotates and zooms in on the image using a high-end imaging software called IMARIS.
Next door, on a typical day, researchers from Cincinnati Children’s or the University of Cincinnati find their seats. Plush black curtains divide the work areas to block stray light. Careful temperature control protects sensitive samples. Here, deep within the research complex at our Burnet Campus, scientists gather to share access to seven powerful Nikon confocal microscopes. These amazing devices can capture images all the way down to the subcellular level. But what makes this space so valuable to scientists is not just the high-end microscopes. It is the image analysis tools, the training, and the methodologies used to interpret the images that make this shared facility special.
Center of excellence provides strong support
“Quantitative analysis of pixel data is important because journals don’t just accept a nice picture of a phenotype or cell,” says Matthew Kofron, PhD, the CIC’s Director. “Proper analysis of data is essential for highly competitive publication and funding. So while a picture is worth a thousand words, a graph with error bars is worth a thousand pictures.”
The CIC here is a Nikon Center of Excellence. As such, the company not only provides top-of-the-line tools, but also ongoing training and support. The assistance ranges from initial training in microscopy to troubleshooting equipment to setting up protocols that allow researchers to get good, reproducible data. The CIC opened in 2012 and operates 24/7 for badged personnel. Since its grand opening, nearly 400 users from more than 120 labs have used the facility. Their combined work exceeds 1,000 hours per month.
Bringing color & dimension into imaging
At one of the microscope stations, CIC manager Michael Muntifering calls up a slide image. The screen shows a mouse hippocampus represented by 9,000 bright green dots—each dot, a cell.
In some cases, proteins autofloresce in tissues. In others, researchers use fluorescently labeled antibodies to stain structures of interest. The confocal microscopes utilize lasers to excite those florescent molecules. As the molecules light up, a series of filters separates different wavelengths into color channels that allow differential labeling of cells of interest. In single-photon confocal microscopy, an advanced method available only at some research centers, a pinhole eliminates out-of-focus light from thick samples. This allows researchers to optically section samples for 3-D reconstruction.
After receiving training from the CIC, Cincinnati Children’s researcher Sean McGrath used the microscope for a study published in 2015 in the journal Diabetes. Using images and cell counting data gathered at the CIC, McGrath found that NEUROG3 is essential for endocrine pancreas development in humans and that as little as 10 percent NEUROG3 is enough to form pancreatic endocrine cells.
From a single dot, knowledge grows
Next, Muntifering brings up a single, living cell on the monitor. “This technology allows us to see exactly where individual molecules are.” He points to one dot on the screen that seems to blink off and on. That tiny blink demonstrates a quantum mechanics phenomenon called triplet state excitation. This is the backbone of a method for subcellular resolution called stochastic optical reconstruction microscopy. The blinking dot serves as a field marker that allows researchers to locate that molecule across multiple frames of the same cell. One dot, fluttering in an ocean of data points. That’s how deeply the CIC can help scientists dive into their work.
(This article originally appeared in our 2016 Research Annual Report)