New Mouse Model Enables Discovery of Potential Treatment for Rare Vascular Tumors—and Beyond
Research By: Sandra Schrenk, PhD | Elisa Boscolo, PhD
Post Date: April 24, 2023 | Publish Date: April 6, 2023
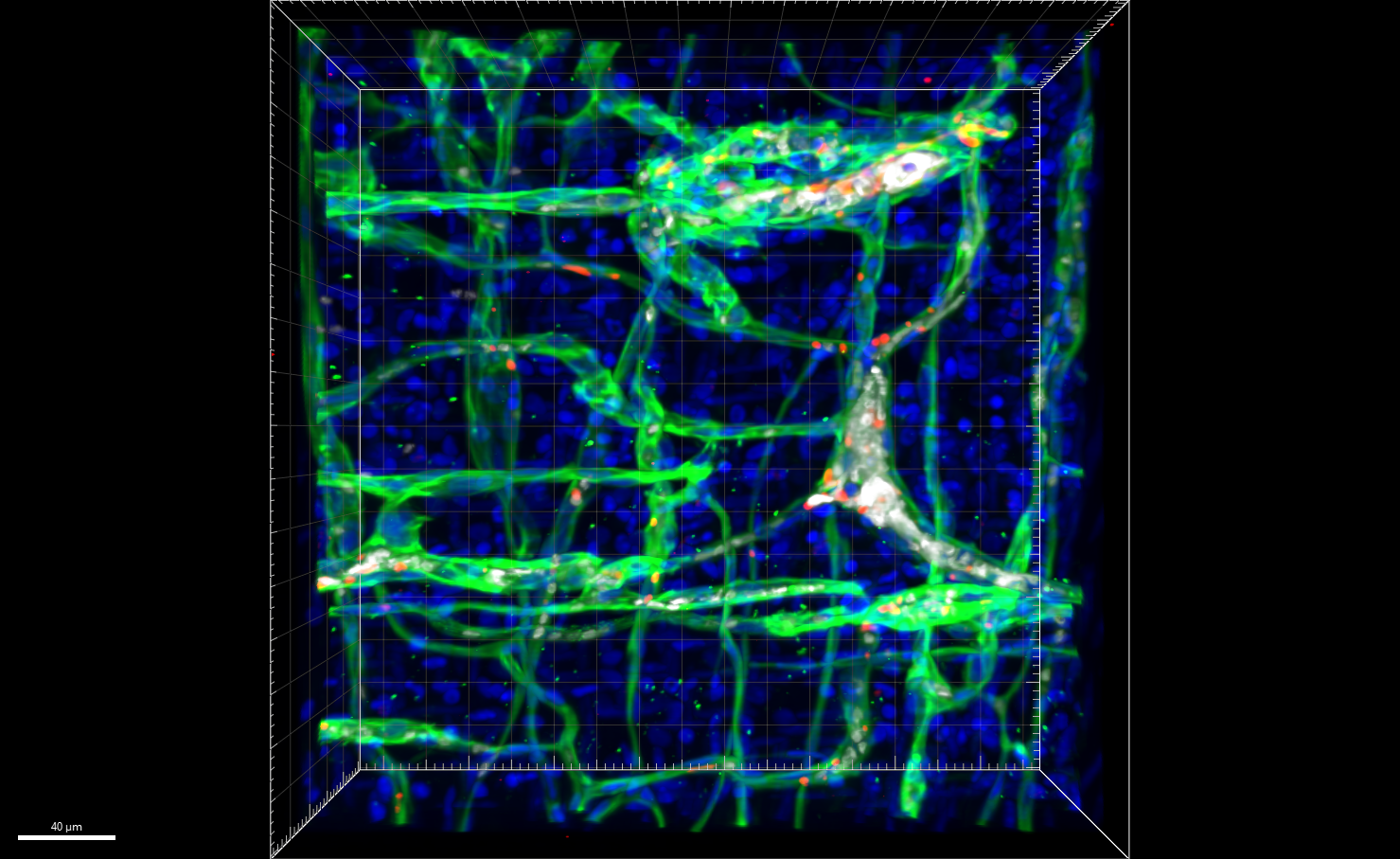
In mice expressing hyperactive GNAQ mutations, trametinib suppresses tumor growth when a rare vascular condition strikes. These findings may also help address more common conditions.
When infants or children are affected by a class of rare, fast-growing vascular tumors associated with a mutation in the gene GNAQ, they experience extensive disfigurement, chronic pain and a life-threatening complication known as the Kasabach-Merritt phenomenon (KMP). A lack of animal models to study this disease process has hampered research to find effective, non-invasive treatments.
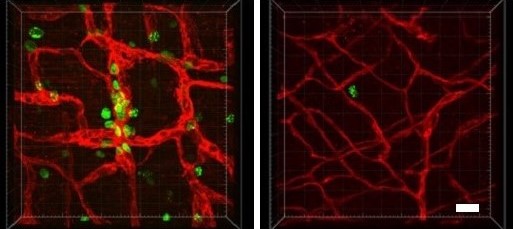
Now, for the first time, experts at Cincinnati Children’s have developed a genetically modified mouse that mimics the formation of these vascular tumors, including the KMP complication.
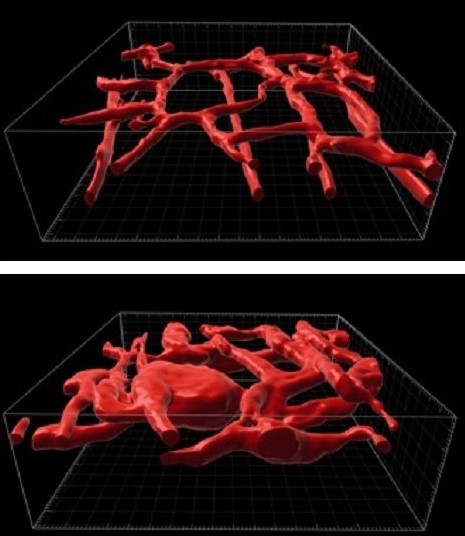
Further, by studying this animal model, the team has discovered a treatment that may eventually help treat affected children. Specifically, they found that when mice mimicking the human condition are treated with trametinib—a MEK inhibitor often used to treat melanoma—their tumors shrink and their survival is significantly extended.
Details were published April 6, 2023, in Nature Communications. The research was led by first author Sandra Schrenk, PhD, and corresponding author Elisa Boscolo, PhD, both from the Division of Experimental Hematology & Cancer Biology at Cincinnati Children’s.
What is the Kasabach-Merritt phenomenon?
KMP is an extreme and potentially lethal consequence triggered by two related diseases that cause fast-growing vascular tumors: tufted angioma (TA) and Kaposiform hemangioendothelioma (KHE).
When these diseases develop, patchy skin discolorations can rapidly swell into massive reddish-brown tumors that distort the affected area, such as the head and neck, a leg, a shoulder, or a portion of the trunk. When these fast-growing vascular tumors form, they can sequester platelets from the bloodstream, which in turn interferes with blood clotting in non-affected parts of the body and raises the risk of serious bleeding. The mortality risk in patients with KMP is as high as 30%.
The precise number of children affected by this condition is not known, but is currently believed to be between 1 in 100,000 and 1 in 700,000 births in the US. Cincinnati Children’s—one of the nation’s top pediatric cancer centers—has treated about 15 cases in the past three years.
Treatment for these children has varied among medical centers. A high risk of bleeding complications makes surgery a poor option and there are no FDA-approved medications for these conditions.
In some cases, patients have shown partial response to sirolimus—an mTOR inhibitor used most commonly as an immunosuppressant. However, the mechanism of action of sirolimus in these patients has not been clear. Now, the research led by Boscolo and colleagues suggests that a drug targeting the MAPK pathway might be more effective than sirolimus.
“Our findings could lead to improved treatments because this therapy acts specifically on the pathways that are activated as consequence of the GNAQ mutation,” Boscolo says.
While the Kasabach-Merritt phenomenon is very rare, this discovery also suggests new approaches for treating other vascular anomalies associated with mutations affecting the MAPK pathway, such as arteriovenous malformations and kaposiform lymphangiomatosis, Boscolo says.
Next steps
While this article demonstrates in vivo effectiveness of trametinib in mice, the co-authors note that RNA sequencing data suggests that trametinib treatment normalized the expression of 73 genes while 544 remained elevated. Future studies should focus on these genes to identify other targets that could be used in combination with MAPK inhibition.
“The model we report here should be instrumental for testing novel targeted therapeutic strategies for the treatment of patients affected by GNAQ-related vascular malformations and tumors,” Boscolo says.
About the study
Co-authors from Cincinnati Children’s and the University of Cincinnati included first author Sandra Schrenk, PhD, and contributors Lindsay Bischoff, Jillian Goines, MS, Yuqi Cai, PhD, Shruti Vemaraju, PhD, Yoshinobu Odaka, PhD, Samantha Good, MS, Sara Szabo, MD, Damien Reynaud, PhD, Joseph Palumbo, MD, and Richard Lang, PhD.
Contributing co-authors also included Catherine Van Raamsdonk, PhD, from the University of British Columbia in Canada.
Funding sources for this work included the National Heart, Lung, and Blood Institute (R01 HL117952), the National Center for Advancing Translational Sciences (UL1TR001425-05A1), the National Health Institutes (U54 DK126108), the Charlotte R. Schmidlapp Women Scholars program at Cincinnati Children’s, and the American Heart Institute (833891).
| Original title: | MEK inhibition reduced vascular tumor growth and coagulopathy in a mouse model with hyperactive GNAQ |
| Published in: | Nature Communications |
| Publish date: | April 6, 2023 |
Research By


Our research focuses on modeling vascular anomalies with the goal of identifying genes and signaling pathways crucial for the formation of vascular anomalies.