It’s Safe to Give COVID-19 and Influenza Vaccines Together
Post Date: November 18, 2024 | Publish Date: Nov. 6, 2024
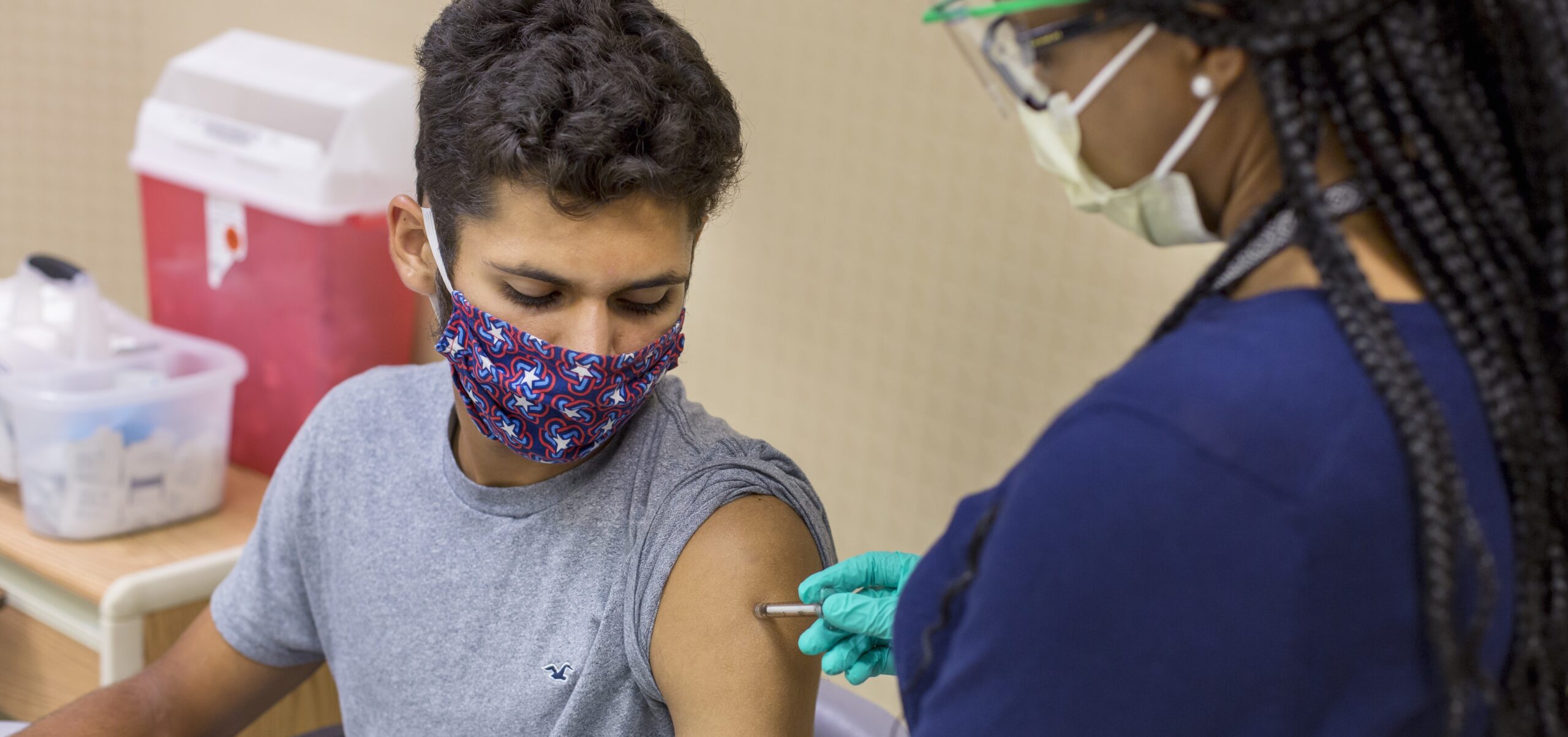
Cincinnati Children’s was the lead enroller of participants in a recent multi-site clinical trial that found it is safe to administer at the same time vaccines that can help prevent serious illness from COVID-19 and the flu.
The findings, published Nov. 6, 2024, in JAMA Network Open, support the option of getting influenza and COVID-19 vaccines together, making it easier to protect oneself against both of these respiratory viruses during the winter months.
Elizabeth Schlaudecker, MD, MPH, and Mary Allen Staat, MD, MPH, of the Division of Infectious Diseases at Cincinnati Children’s collaborated on the study with colleagues at the Centers for Disease Control and Prevention and two other CDC-sponsored Clinical Immunization Safety Assessment Project sites, Duke University and Johns Hopkins University.
The 335 participants in the study conducted at the three sites between Oct. 8, 2021, and June 14, 2023, were non-pregnant people 5 years of age or older.
Now, the Cincinnati Children’s researchers are enrolling participants who are pregnant in a similar study of the safety of administering the vaccines together.
The ultimate goal, Schlaudecker says, is to provide high levels of vaccination coverage during anticipated periods of high influenza and SARS-CoV-2 virus transmission, protecting those who need it the most.
“Getting a flu vaccine has been an annual routine for most of us for many years, as we know that it is an effective way to prevent severe influenza illness,” says Schlaudecker, who noted that flu vaccines require yearly updates because of changes in the viruses.
When confronted by the COVID-19 pandemic, the vaccines developed in response were a lifesaving intervention that first became available in December 2020. But COVID-19 vaccines have similarly required updates to adapt to changes in the SARS-CoV-2 virus and to continue to provide protection against COVID-19 infection.
Because influenza, SARS-CoV-2 and other respiratory viruses often peak in the winter months, people usually get the influenza and SARS-CoV-2 vaccines around the same time during the late summer and early fall. To make it easy and convenient for people to get these vaccines, the CDC Advisory Committee on Immunization Practices’ guidance allows for simultaneous administration of the vaccines at two different anatomic sites.
“However, because the vaccines were not given together during the vaccine trials, we have not had a lot of information about the safety of administering the two vaccines together,” Schlaudecker says.
In this clinical trial, titled “Safety of Simultaneous vs. Sequential mRNA COVID-19 and Inactivated Influenza Vaccines,” 169 participants were randomized to the simultaneous group (received influenza and mRNA COVID-19 vaccines on Day 1 and placebo vaccine eight to 15 days later), and 166 participants were randomized to the sequential group (received placebo and mRNA COVID-19 vaccine on Day 1 and influenza vaccine eight to 15 days later).
The study team monitored for reactions and adverse events on Days 1 through 7 for each group. Reactions included pain and swelling at the injection site, as well as symptoms such as fever and chills. The percentage of participants experiencing reactions after vaccinations was comparable in the group who received vaccines simultaneously versus the group who received the vaccines eight to 15 days apart.
All reactions reported after vaccination were short-lived, and no participant sought medical care for any of the reactions in either study group.
Read more about vaccine research at Cincinnati Children’s
| Original title: | Safety of Simultaneous vs Sequential mRNA COVID-19 and Inactivated Influenza Vaccines: A Randomized Clinical Trial |
| Published in: | JAMA Network Open |
| Publish date: | Nov. 6, 2024 |







