How Some Liver Cells Switch Identities to Build Missing Bile Ducts
Research By: Stacey Huppert, PhD
Post Date: July 2, 2019 | Publish Date: May 2018
“We have known for a long time that the liver has more ability to regenerate than other organs. Only recently have we had the tools to study this ability in depth. Now we have a high-level understanding.”
—Stacey Huppert, PhD
The liver is an amazing organ. Every day, it takes a beating as it processes everything from medications to alcohol consumption. All these insults have prompted the liver to develop a rapid healing ability that does not rely on stem cells.
Now a breakthrough study has revealed how the organ rebuilds itself with such speed. Instead of relying upon stem cells to ramp up production when disease or injury causes a shortage in one critical type of liver cell, the organ can instruct another type of liver cell to simply change identities.
“Previous research has detected adaptive reprogramming in other organs, but it typically involves only a few cells at a time. Our study shows that cells switch their identity at a massive rate in the liver,” says Stacey Huppert, PhD, a developmental biologist in the Division of Gastroenterology, Hepatology and Nutrition and one of two leading co-authors of the paper.

THE RARE DISEASE ALGS OFFERS INSIGHTS
“We have known for a long time that the liver has more ability to regenerate than other organs. Only recently have we had the tools to study this ability in depth. Now we have a high-level understanding,” Huppert says.
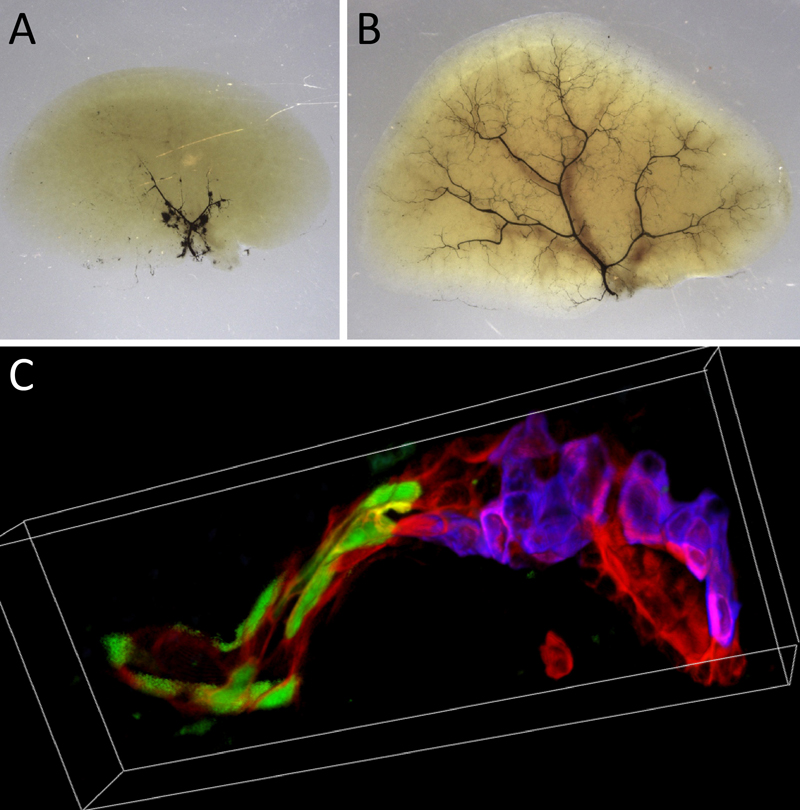
To explore the process, the team worked with mice bred to mimic Alagille syndrome (ALGS), a rare genetic disorder linked to deficiencies in the Notch pathway. People born with ALGS can have too few bile ducts, excessively narrow ducts and/or entire missing branches of the biliary tree.
“We have known for a long time that the liver has more ability to regenerate than other organs. Only recently have we had the tools to study this ability in depth. Now we have a high-level understanding.”
Like patients with severe ALGS, mice bred to lack duct-forming cholangiocytes quickly developed signs of liver injury after birth. However, over time the mice’s symptoms improved because hepatocytes converted into cholangiocytes and formed fully functional bile ducts.
Huppert and colleagues determined that the cell-type switch occurs by supplanting the Notch pathway with a pathway regulated by transforming growth factor beta (TGFb), another protein that controls cell growth.
Previous research had confirmed that cholangiocytes also can transform into hepatocytes when needed. These findings suggest a level of flexibility that offers an opportunity to address multiple liver diseases, the study authors say. It may become possible to “retrofit” biliary systems in damaged organs.
Discovering this phenomenon and learning how it works took nearly five years. The team included co-first authors Johanna Schaub and Simone Kurial from UCSF and Kari Huppert from Cincinnati Children’s.
However, much more work lies ahead to produce a viable treatment for human disease.
NEXT STEPS
Huppert presented the latest findings in June 2018 in Washington, DC, at Digestive Disease Week, a major meeting organized by the American Society for Gastrointestinal Endoscopy. Huppert also has been invited to speak in early 2019 at scientific meetings in Florida and Colorado.
The research also will be discussed at the annual Alagille Syndrome Scientific Meeting, to be held in June 2019 at Cincinnati Children’s.
“We are working on the mechanisms behind hepatic cell plasticity to enable safe and regulated in vivo reprograming of hepatocytes to cholangiocytes,” Huppert says. “Additionally, we are digging deeper into the potential of hepatocyte transplantation and the extrinsic cues that drive hepatocyte to cholangiocyte transdifferentiation.”
| Original title: | De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation |
| Published in: | Nature |
| Publish date: | May 2018 |
Research By