First Treatment for Eosinophilic Esophagitis Wins FDA Approval
Post Date: May 20, 2022 | Publish Date:

Experts at Cincinnati Children’s are savoring the news that the U.S. Food and Drug Administration approved Dupixent (dupilumab) on May 20, 2022, as the first treatment for eosinophilic esophagitis (EoE)
EoE is a chronic inflammatory disease driven by type 2 inflammation that damages the esophagus. About 160,000 people have EoE in the U.S.
People with EoE can have painful choking experiences when trying to swallow even small amounts of food. Managing the condition often involves following highly restrictive diets, with some relying on feeding tubes. Until now, people have also received various therapies in clinical trials and off-label uses of drugs approved for other conditions.
Read the FDA announcement
Dupilumab is a monoclonal antibody that helps inhibit the inflammation associated with EoE. The therapy is now approved for use in adults and pediatric patients 12 years and older weighing at least 40 kilograms (about 88 pounds).
Approval a Milestone in 20-Year Research Quest
“This is a victory for science, patients, rare diseases and advocacy,” says Marc Rothenberg, MD, PhD, director of the Cincinnati Center for Eosinophilic Diseases at Cincinnati Children’s.
Rothenberg has been researching EoE for the past two decades and has played an essential role in dupilumab’s development for EoE.
Early on, the condition was not well-understood. Some thought it might be related to acid reflux disease. However, studies by Rothenberg and his team demonstrated that EoE was mediated by an allergic response to food, and the disease mechanism involved a dysregulated immune response driven by interleukin (IL-13) elicited inflammation.
Rothenberg and his team conducted proof-of-principle studies, initially in pre-clinical systems and subsequently in patients, which provided the scientific rationale and proof for using dupilumab to treat patients with EoE. The therapy works by blocking the binding of IL-13 and a related protein (interleukin-4) to its receptor, which curtails the allergic arm of the immune system.
Rothenberg continues to lead studies of EoE and related conditions as principal investigator of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR), which is part of the NIH-funded Rare Diseases Clinical Research Network (RDCRN).
Investigators in CEGIR’s national network of 20 clinical sites have worked collaboratively to generate or refine several tools that support the clinical trial process. These tools, which measure disease severity and treatment efficacy, include collecting patient-reported outcomes, endoscopic reference scores, a histologic scoring system, and an EoE diagnostic panel.
Read Clinical Trial Details in Announcement from Regeneron Pharmaceuticals, Inc., and Sanofi
NEXT STEPS
Cincinnati Children’s follows and studies data from more than 2,500 patients from across the United States who suffer from eosinophilic disease. The new treatment will be available at Cincinnati Children’s and Rothenberg predicts usage will grow soon.
See a Facebook note from Dr. Rothenberg sharing more information with patients
“This treatment will benefit about one third of the current 160,000 people diagnosed in the United States. However, most patients are not yet using it,” Rothenberg says.
Adding to the excitement: This news arrived just in time to finish out National Eosinophil Awareness Week.
Highlights of a long journey
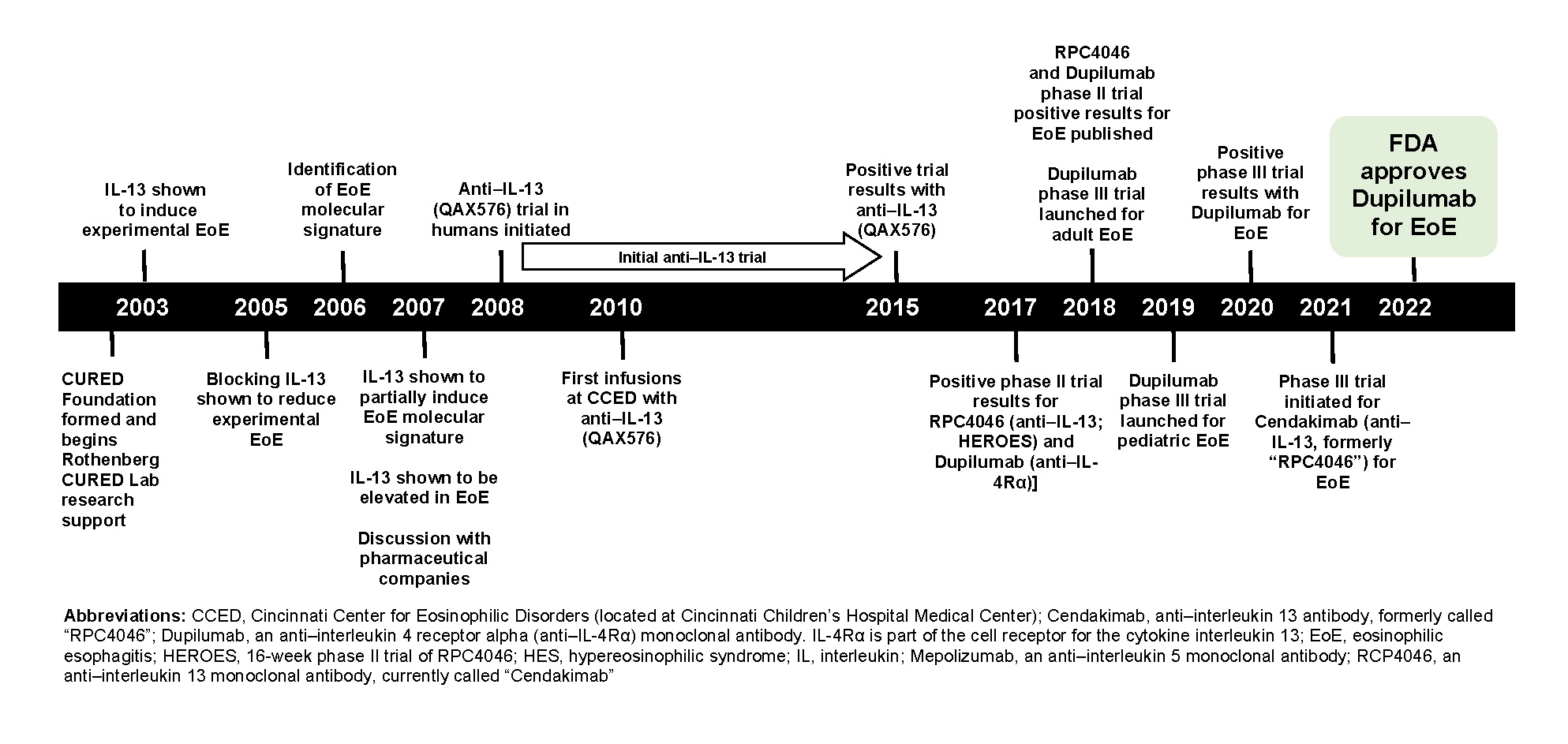
Here are the major advances and achievements from the Rothenberg CURED laboratory that formed the basis for the FDA’s approval of dupilumab: