Discovery about nerve fiber insulation may lead to better treatments for several rare conditions
Research By: Jiajia Wang, PhD | Qing Richard Lu, PhD
Post Date: August 12, 2020 | Publish Date: Aug. 12, 2020
In early-stage study, the experimental drug GSK-J4 appears to prompt myelin sheath repair when EED protein malfunctions
For some diseases affecting the brain and nervous system, it may be possible to coax the body to repair damage that occurs to myelin, a critical layer of insulation that protects the function of nerve fibers.
This finding, from a study led by Cincinnati Children’s scientists Jiajia Wang, PhD, first author, and senior author Qing Richard Lu, PhD, was published online Aug. 12, 2020, in the journal Science Advances.
The researchers report discovering that EED, a polycomb repressive complex 2 (PRC2) protein, plays a deep role in regulating the process of myelin formation—and that the process can be manipulated in a lab setting. In years to come, this discovery could lead to new forms of treatment for diseases including multiple sclerosis (MS), malignant peripheral nerve sheath tumors, brain tumors known as diffuse intrinsic pontine gliomas (DIPG), as well as Weaver-like syndrome, and other rare demyelinating diseases.
“Oligodendrocytes are critical cells for human health because they make up the myelin sheath, which insulates the axon to allow high-speed electrical impulses,” Lu says. “If immature oligodendrocytes don’t differentiate, then they can hinder sheath repair and lead to loss of nerve function in cases such as multiple sclerosis or spinal cord injury. On the flip side, if they divide and proliferate too much they can become tumors.”
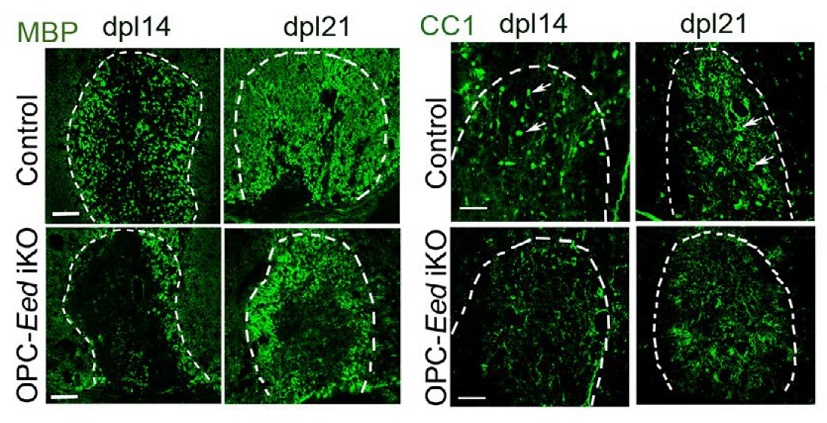
Wang and colleagues conducted a series of experiments to determine the role EED plays in affecting the activity of several more genetic pathways that act in combination to support myelin growth. The new study further reveals that an experimental compound made by GlaxoSmithKline called GSK-J4 might prompt myelin growth when there’s a lack of natural EED activity.
EED regulates the activity of the PRC2 enzyme, which plays a central role in nerve fiber development. A mutant form of the EED gene had been suspected of playing a role in causing Weaver-like syndrome, a very rare condition that results in unusually tall children with widely-spaced eyes, varied levels of intellectual disability, and other unusual features.
“The fact that these patients have defects in myelin and white matter development led us to begin this avenue of research for probing EED function in myelin and white matter development in the brain,” Lu says.
In cells under laboratory conditions, the GSK-J4 drug compound partially restored nerve sheath formation after damage had occurred in cells exhibiting Weaver-like syndrome.
Eventually, this could be good news for those with Weaver-like syndrome. But beyond that group, the team’s work indicates that a treatment affecting the EED/PRC2 pathway could be useful for even more people who are born each year with white matter injuries and diseases as well as those who develop certain oligodendrocyte-related pediatric brain tumors.
While this early sign of success is encouraging, Lu stresses that work has just begun to test the drug in animal models. Only after those tests are complete could clinical trials begin to determine if this drug—or others yet to be developed—might be safe and effective as human treatments.
| Original title: | EED-mediated histone methylation is critical for CNS myelination and remyelination by inhibiting WNT, BMP, and senescence pathways |
| Published in: | Science Advances |
| Publish date: | Aug. 12, 2020 |
Research By