CELA1 Antibody Shows Therapeutic Potential for COPD and Emphysema
Post Date: February 2, 2024 | Publish Date: Jan. 9, 2024
Scientists at Cincinnati Children’s lead a multi-center team that reports a significant advance in pulmonary research
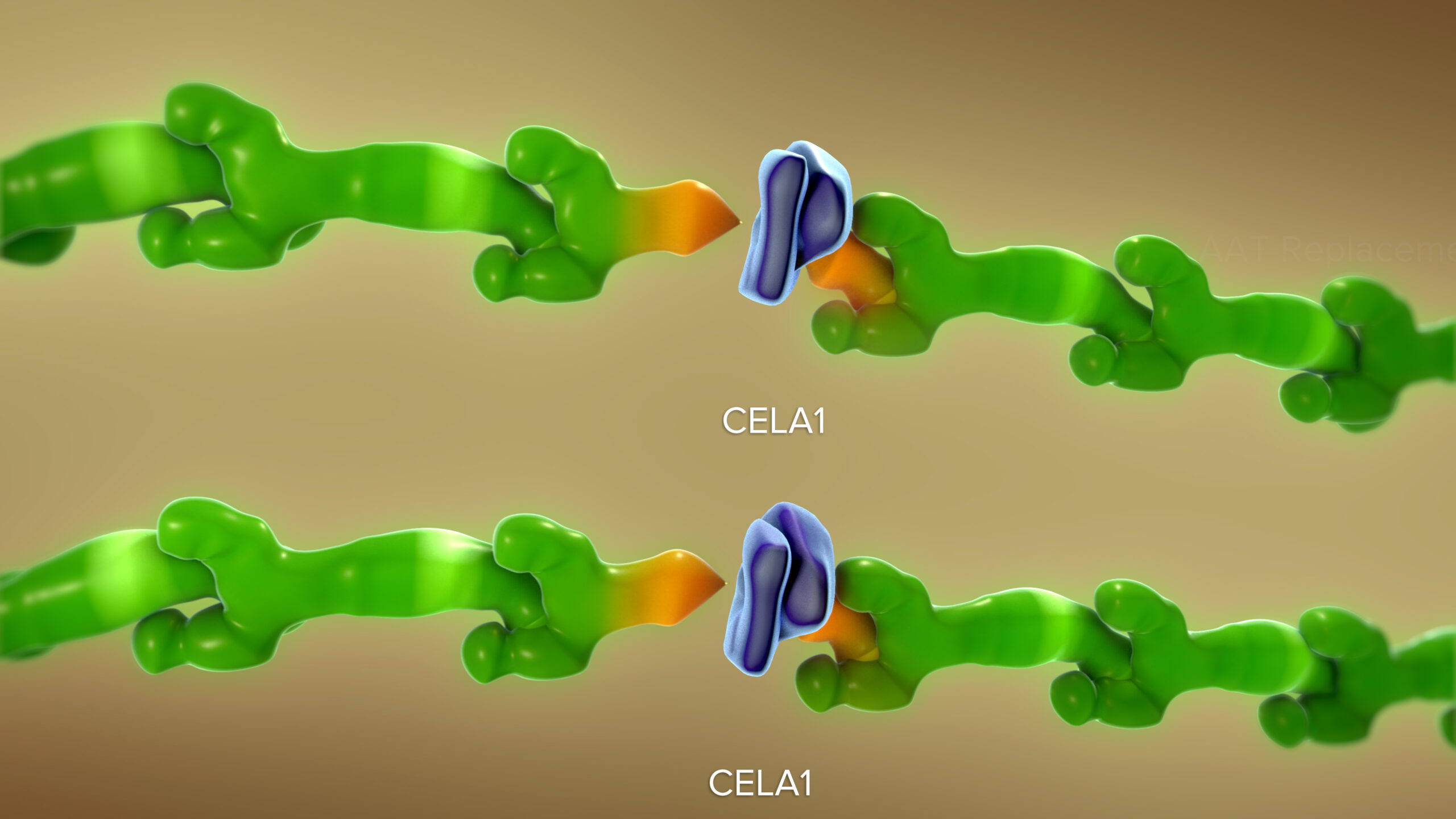
In mice, a novel antibody that blocks chymotrypsin-like elastase 1 (CELA1) successfully reduced the lung damage caused by diseases such as age-related emphysema and chronic obstructive pulmonary disease (COPD), according to a study published Jan. 9, 2024, in JCI Insight.
When CELA1 is introduced to adult lung specimens under physical stretch, it actively bonds to the lung tissue. “This bonding enhances the remodeling of elastase activity, a key disease process in lung disorders,” says corresponding author Brian Varisco, MD, previously with the Division of Critical Care Medicine at Cincinnati Children’s and now vice chair of research at the University of Arkansas for Medical Sciences.
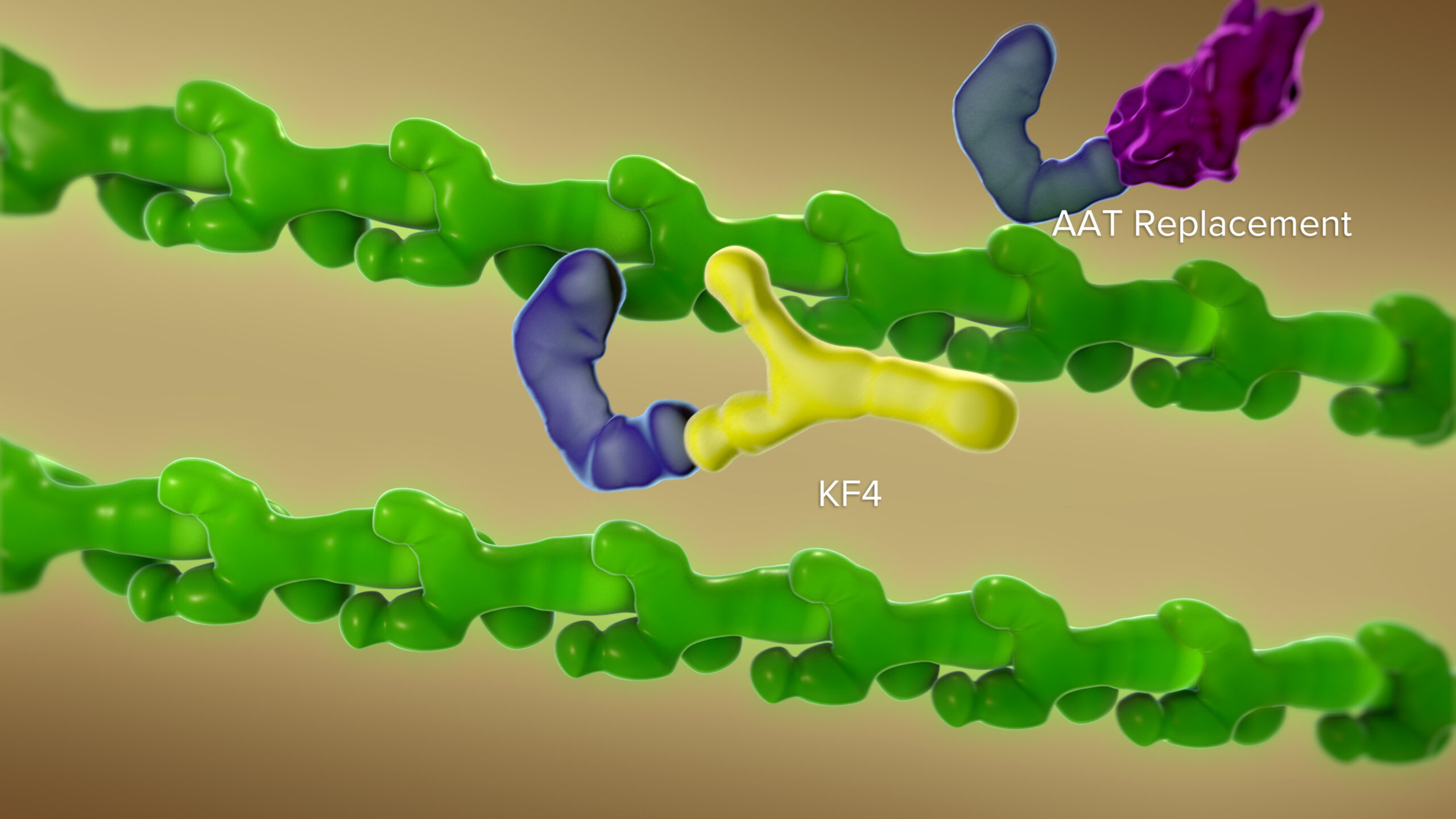
“By using a novel antibody to block CELA1, we observed a protective decrease in elastin remodeling. We were able to duplicate these protective effects in studies involving three types of chronic obstructive pulmonary disease,” Varisco says.
The findings suggest that manipulating CELA1 activity could have therapeutic potential for COPD and other emphysematous lung diseases. Learn more from this video.
“This novel technology represents a promising development in the field of pulmonary medicine,” says Muyang Hu, MS, Innovation Ventures acceleration manager for biologics, cell & gene therapies.
Next steps
Varisco’s research team plans to further clarify that the remodeling process observed in the mouse models also occurs in human tissue. Initially, the plan involves humanizing a mouse antibody for CELA1, which will be produced by a contract research organization and returned for further experimentation. Once developed, it will take about a year to ensure the stability and efficacy of the antibody.
“Once the functionality of the antibody is confirmed, the technology will reach to the next value inflection point towards commercialization,” Hu says.
Cincinnati Children’s Innovation Ventures continues to partner with Varisco to bring this technology to the marketplace.
About the study
In addition to Varisco, Cincinnati Children’s co-authors included Mohit Ojha, MD; Noah J. Smith; Andrew Devine; Rashika Joshi, MD; Emily Goodman; Qiang Fan, PhD; Richard Schuman, PhD; Aleksey Porollo, PhD; James Wells, MD; Etka Tiwary, MD; Matthew Batie; Jerilyn Gray; Hitesh Deshmukh, MD, PhD; Michael Borchers, PhD; and Samuel Ammerman.
Collaborators also included experts from Cincinnati Children’s Division of Critical Care Medicine, Center for Autoimmune Genomics and Etiology (CAGE), Clinical Engineering, Perinatal Institute, and the Center for Perinatal Immunity; Antibody and Immunoassay Consultants (Rockville, MD); Arkansas Children’s Research Institute; Lincoln Medical Center and Mental Health Center (New York, NY); Ohio State University’s School of Medicine; Ohio University’s Heritage College of Osteopathic Medicine; University of Alabama at Birmingham School of Medicine, UAB Lung Health Center; University of Arkansas for Medical Sciences College of Medicine; and the University of Cincinnati’s College of Medicine and the Division of Pulmonary and Critical Care Medicine;.
This study was funded in part by grants from: the Cincinnati Children’s Innovation Fund; an A1 Foundation Research Award (498262); the National Heart, Lung, and Blood Institute (R01HL141229, K08HL131261 and R01HL119538); the Veteran’s Administration (I01BX002347) and the National Institutes of Health (R01HL142708 and R01HL155611).
| Original title: | Anti-CELA1 antibody KF4 prevents emphysema by inhibiting stretch-mediated remodeling |
| Published in: | JCI Insight |
| Publish date: | Jan. 9, 2024 |