Asthma Risk Score Tool Performs Well Across Diverse Populations
Research By: Jocelyn Biagini, PhD | Gurjit Khurana Hershey, MD, PhD
Post Date: August 11, 2023 | Publish Date: Aug. 4, 2023
The ability to predict asthma development earlier in life is a key component to preventing and lessening the burden of a disease that affects roughly six million children in the United States.
In 2018, researchers from Cincinnati Children’s developed the Pediatric Asthma Risk Score (PARS), which uses six factors to predict asthma risk among children between birth and age 3. PARS performed well but was developed in relatively homogenous populations, so its use in a broader context with diverse groups was relatively unknown.
In a paper published Aug. 4, 2023, in NEJM Evidence, Gurji Khurana Hershey, MD, director of Asthma Research, and Jocelyn Biagini, PhD, from Cincinnati Children’s, demonstrate that PARS performed well in determining asthma risk estimates in children from a wide range of ethnicities, backgrounds, and asthma susceptibilities. These findings, importantly, show the generalizability of PARS and demonstrate that PARS can be used to identify young children with low to high risk for asthma. It can be used to better inform parents and pediatricians to provide care for the child and can be used to populate clinical trials to test novel prevention strategies.
“We developed PARS to give families and clinicians a more personalized estimate of a child’s risk of developing asthma,” Khurana Hershey says.
Prior to PARS, the Asthma Predictive Index (API) was used as the primary method of determining which children were most likely to develop asthma later in life. The API is dichotomous tool providing either a “yes” or “no” answer for asthma likelihood, being most effective for children who either demonstrate a high amount of risk, or very low risk. In contrast, PARS is a continuous risk score that provides a risk estimate across the risk spectrum.
Experts at Cincinnati Children’s saw an opportunity to help those children who fall in the middle – by creating a tool capable of predicting mild, moderate and severe asthma risk and enabling families and providers to have a better idea of appropriate interventions. Further, children with lower risk may be more amenable to preventive strategies.
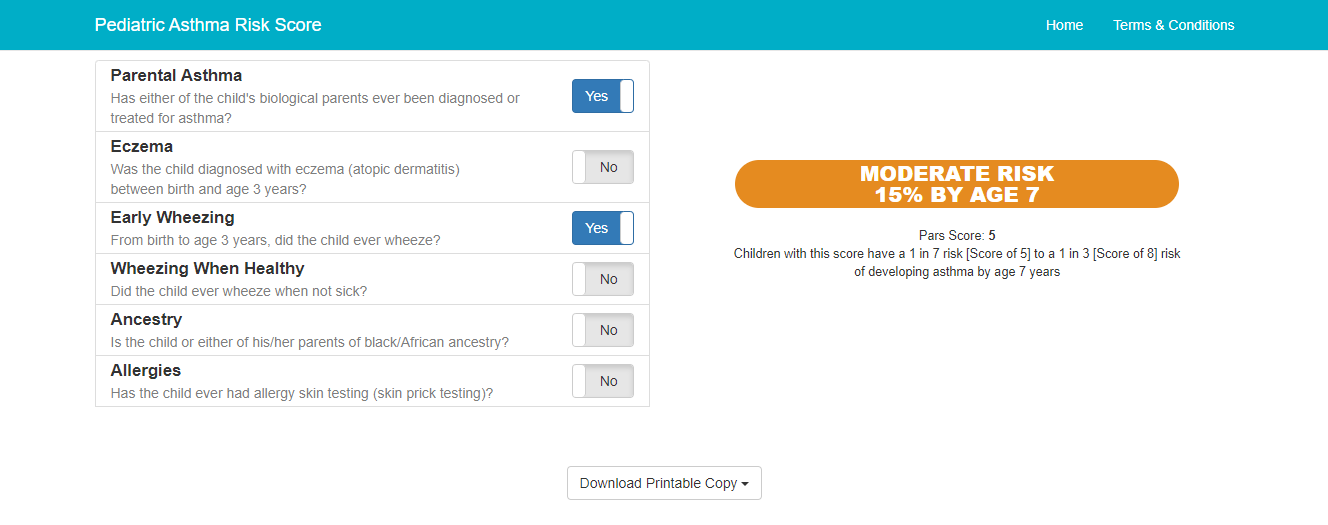
PARS is a free, six-question tool available either as a website, or app downloaded via the Apple Store or Google Play. Risk is assessed by answering “yes” or “no” to six questions ranging from whether a child’s parents have been diagnosed or treated for asthma to allergy testing and ancestry. The tool is simple enough for relatively anyone to use and can help inform parents about their child’s asthma risk.
To date, more than 33,200 clinicians, parents, students and researchers have accessed PARS in more than 160 countries, and the app has been downloaded more than 3,500 times.
PARS was originally developed using two cohorts, one in Cincinnati, which was reflective of the racial diversity of the region (23% self-reported Black and 77% self-reported White), and the Isle of Wight, which was largely homogeneous. To test the generalizability, Cincinnati Children’s researchers leveraged the U.S. National Institutes of Health Environmental Child Health Outcomes – Children’s Respiratory and Environmental Workgroup (ECHO-CREW), a consortium with 13 separate cohorts designed to study asthma across the United States. Ten of the 13 cohorts had data that could be input into PARS from heterogenous and diverse populations that captured more racial, ethnic, and temporal diversity than was included when PARS was first created.
Ultimately, the researchers were able to show that PARS performed equally well amongst all cohorts – roughly 5,600 children – no matter the diversity in the populations. Some cohorts had a high number of African American or Hispanic participants. Some were general risk cohorts while others were high risk cohorts for asthma and allergy development. Across the board, there were no differences in the ability for PARS to provide robust estimates even when there was missing data. Further, the study validated the use and utility of the 6 factors that make up the PARS.
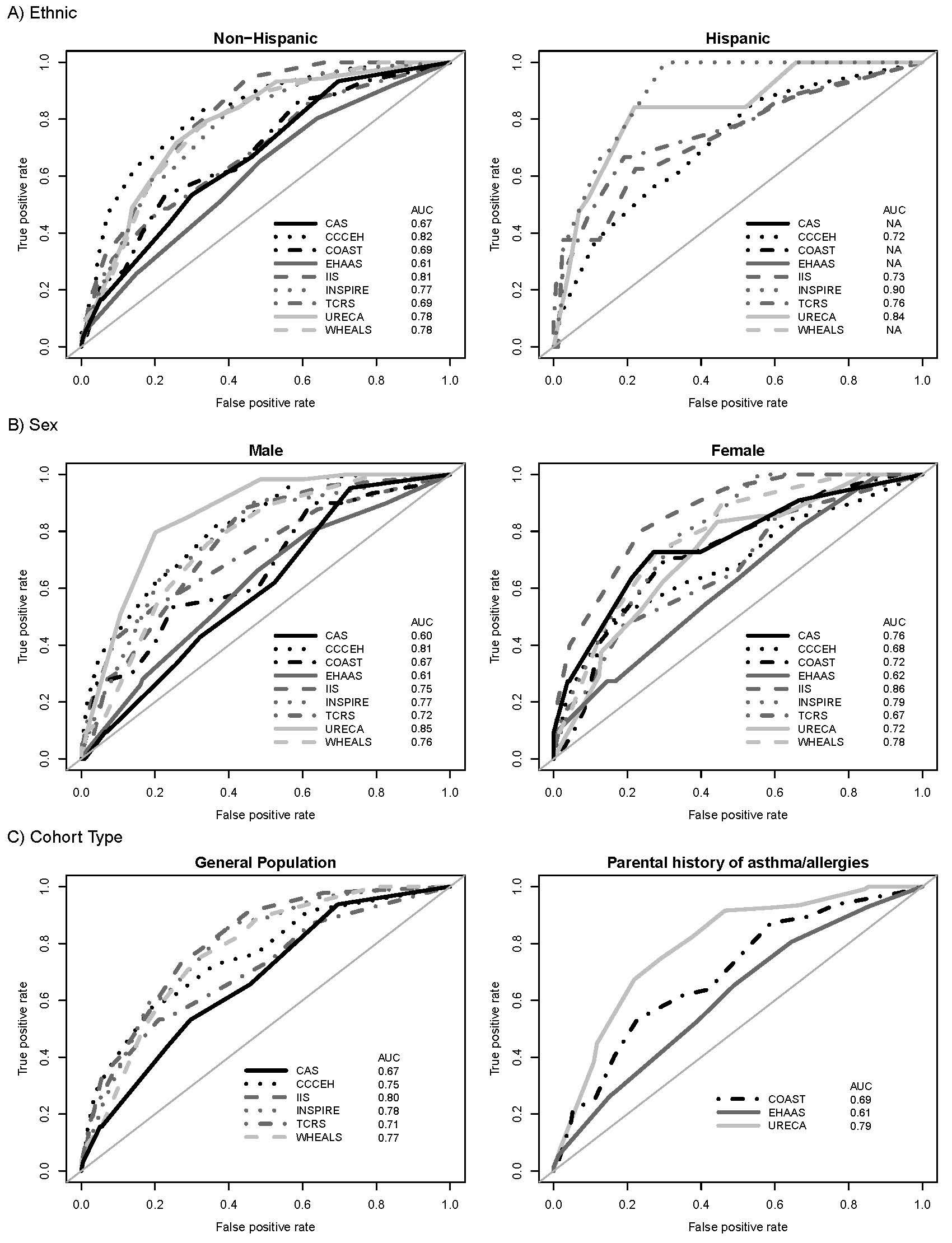
“This analysis enabled us to show that PARS is generalizable across diverse populations, which broadens the utility of the tool to predict asthma in young children across the United States and worldwide,” Biagini says.
Researchers hope that this tool becomes not only the standard for families and providers, but that it will be a critical asset in future clinical trials, which are now primarily populated by participants determined to be at risk for asthma via the API. By utilizing PARS, trials will be able to include children across the globe with more mild-moderate risk factors, testing therapies across the spectrum, with the goal of ultimately preventing these children from developing asthma.
| Original title: | Performance of the Pediatric Asthma Risk Score across Diverse Populations |
| Published in: | NEJM Evidence |
| Publish date: | Aug. 4, 2023 |
Research By


My lab combines epidemiologic, basic, translational and clinical research approaches to answer fundamental questions related to childhood asthma, atopic dermatitis, food allergy and allergic rhinitis.