DWORF Gene Therapy Shows Early Promise for Treating Heart Failure
Research By: Cat Makarewich, PhD
Post Date: December 4, 2025 | Publish Date: Sept. 5, 2025
In mice, treatment restores healthy calcium cycling and boosts mitochondrial function
A team of scientists at Cincinnati Children’s reports success in a mouse model at using a novel gene therapy to improve cardiac function during heart failure—and has launched efforts to continue the research.
In the United States alone, heart failure affects nearly 6.7 million adults and causes more than 450,000 deaths a year. Meanwhile, up to 14,000 U.S. children—often born with heart defects—are hospitalized with heart failure each year. While many treatments are used to help manage heart failure symptoms, no available therapies directly correct the underlying defects in cardiac function.
Now experts report in the journal Circulation Research that a gene therapy centered on a cardiac microprotein called DWORF eventually may transform heart failure treatment.
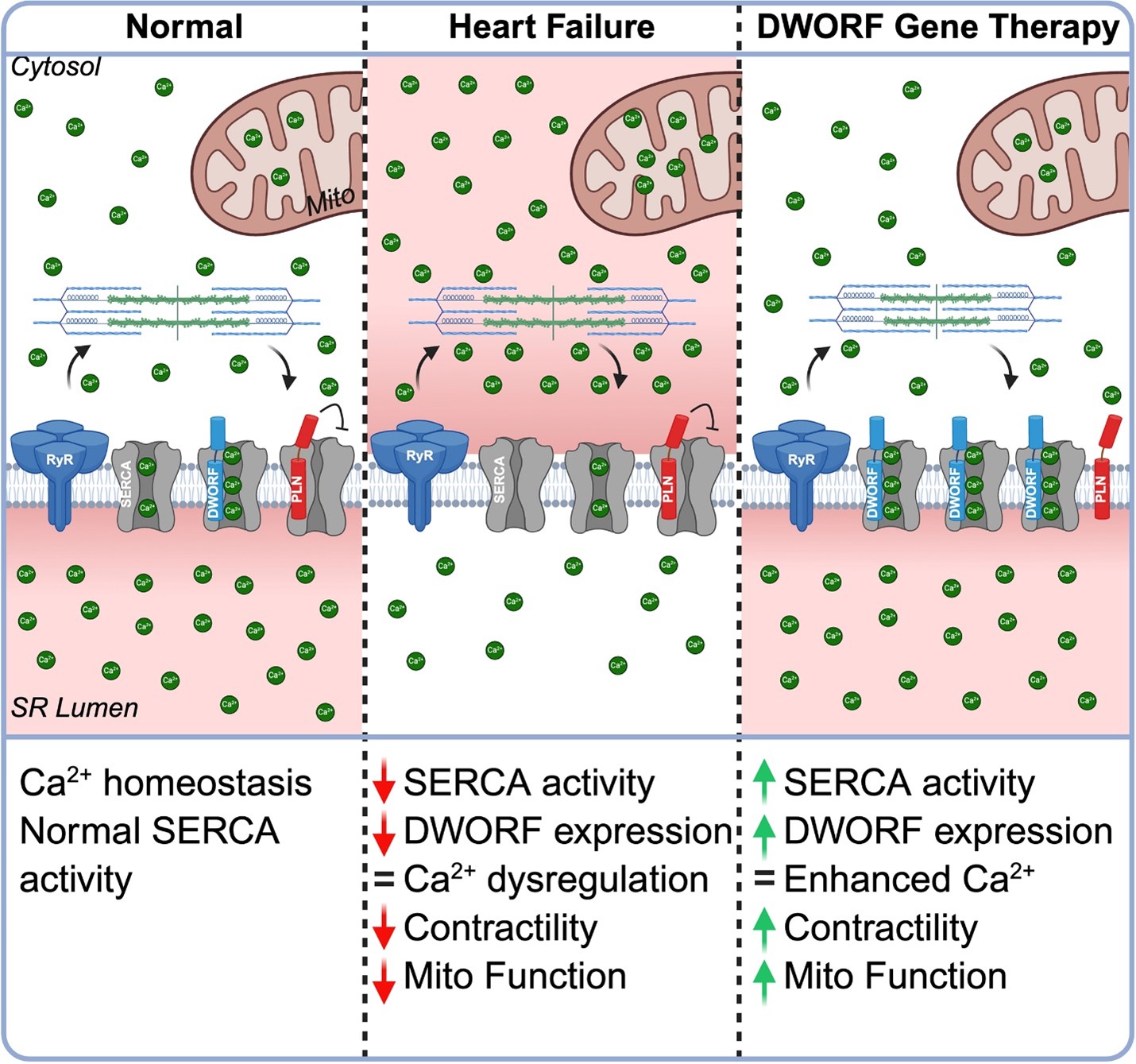
“One of the core problems in heart failure is that heart muscle cells can’t handle calcium properly, which disrupts both the heart’s ability to contract and its cellular energy supply,” says corresponding author Cat Makarewich, PhD. “Our study shows that a newly discovered protein called DWORF, delivered through gene therapy, can fix both of these problems by restoring calcium balance and boosting mitochondrial energy production. This dual benefit is something no current heart failure therapy offers.”
Addressing the Root of Heart Failure
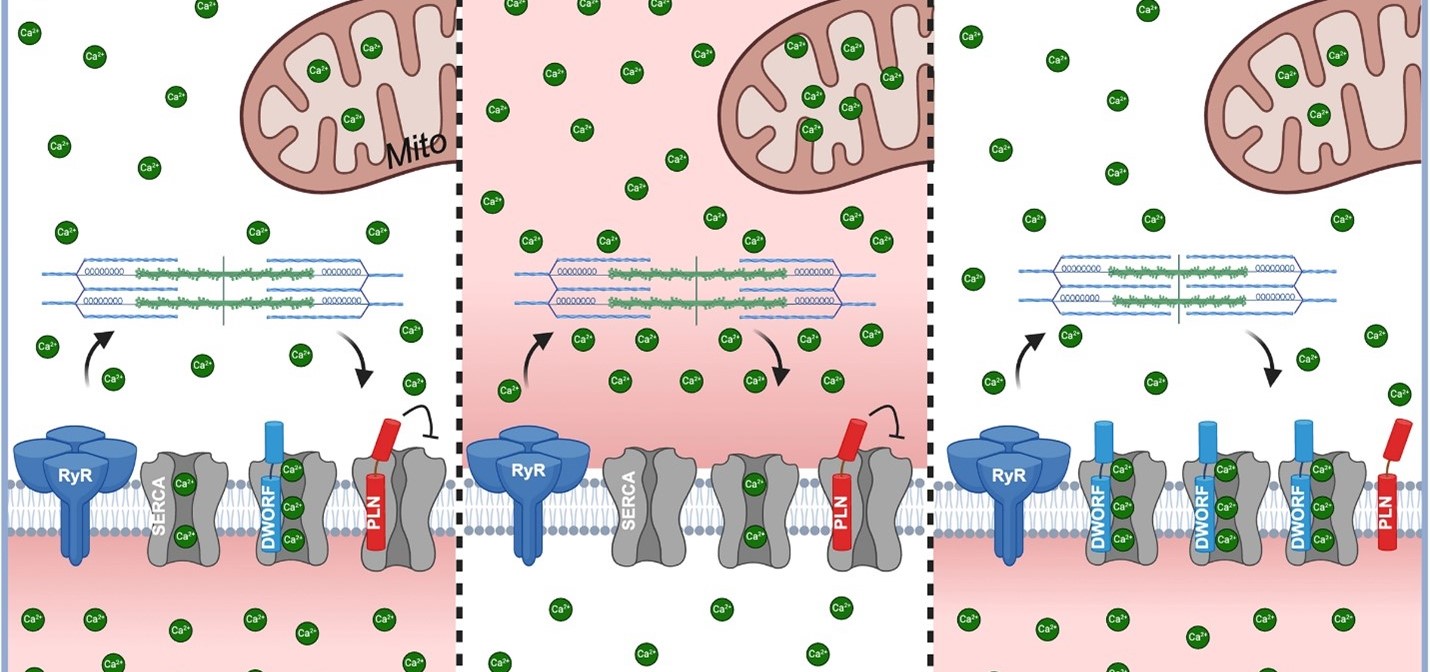
Scientists have understood for more than a decade that many cases of heart failure reflect the diminished function of the protein SERCA2a, which acts as a calcium pump that recharges the heart’s sarcoplasmic reticulum (SR) during each heartbeat. But early attempts to restore calcium cycling have shown limited clinical success.
In 2016, Makarewich, now a member of the Division of Molecular Cardiovascular Biology at Cincinnati Children’s, co-discovered the DWORF microprotein during her postdoctoral fellowship at UT Southwestern. Since then, she has been studying how DWORF directly activates SERCA2a and displaces its natural inhibitors. She and her colleagues discovered that increasing DWORF levels in the heart not only improves calcium handling but also enhances mitochondrial function—the cell’s energy powerhouse.
“The idea of using DWORF as a gene therapy grew directly out of those early mechanistic studies. From initial discovery to this therapeutic proof-of-concept, the work has taken nearly a decade and has involved many collaborators, new technologies, and a sustained commitment to the science,” Makarewich says.
Dual Mechanism: Calcium Cycling and Mitochondrial Boost
Using both transgenic mice and a next-generation gene therapy vector (myo-adeno-associated virus, or MyoAAV), the researchers overexpressed DWORF in the heart. They found that DWORF overexpression led to:
- Enhanced mitochondrial respiration and increased spare respiratory capacity
- Elevated levels of active pyruvate dehydrogenase (PDH), a key enzyme for energy production
- Improved mitochondrial calcium uptake kinetics
These changes translated into better heart function and resilience. In a mouse model of pressure overload–induced heart failure, DWORF gene therapy preserved left ventricular function, reduced harmful cardiac remodeling, and maintained mitochondrial capacity. Notably, these benefits were observed whether the therapy was delivered preventively or after heart failure had already developed.
A New Therapeutic Avenue
DWORF is part of a new wave of gene therapies that aim to fix the root causes of heart failure instead of just treating symptoms.
“Other gene therapies have been tested, including one that delivered SERCA2a directly, but those approaches faced challenges because of the size of the gene and inefficient delivery.” Makarewich says. “DWORF is much smaller and works by activating the patient’s own SERCA2a, which makes it easier to deliver and potentially safer.”
If successful in humans, this therapy could benefit many people because calcium handling defects are common among heart failure patients.
Looking Ahead
Makarewich plans to present this research at the Keystone Conference, “Cardiometabolism in Health and Disease,” in January.
Further next steps include optimizing the dose, conducting additional safety studies in larger animal models, and preparing for regulatory approval to begin testing DWORF therapy in patients.
“We are also working on strategies to improve gene expression levels and exploring combination approaches that deliver DWORF along with SERCA2a to enhance therapeutic benefit,” Makarewich says.
About the Study
Cincinnati Children’s contributors included first author Omar Brito-Estrada, PhD, and Yasuhide Kuwabara, PhD, Aaron Gibson, BA, Keira Hassel, PhD, Michael Kamradt, PhD, Joseph Verry, BME, N. Scott Blair, BS, Michael Bround, PhD, Jiuzhou Huo, PhD, and Jeffery Molkentin, PhD.
Funding sources for this study included grants from the National Institutes of Health (R01HL171221, R01HL160569, R01HL132831, R00AR078253, and F30HL172585); the American Heart Association (PRE1020028 and CDA1274099); and the Cincinnati Children’s Research Foundation.
Don’t Miss a Post:
- Subscribe to the Research Horizons Newsletter
- Follow Cincinnati Children’s Research Foundation on Bluesky, X and LinkedIn
| Original title: | DWORF Gene Therapy Improves Cardiac Calcium Handling and Mitochondrial Function |
| Published in: | Circulation Research |
| Publish date: | Sept. 5, 2025 |
Research By

Our lab investigates the molecular mechanisms that underlie the pathophysiology of cardiovascular disease and skeletal muscle disorders.