The Long Journey of a Small Molecule of Hope
Research By: Daniel Starczynowski, PhD
Post Date: December 5, 2024 | Publish Date:
A groundbreaking clinical trial for the drug KME-0584 may begin in early 2025, offering new hope to people with relapsed AML and high-risk MDS. Reaching this rare milestone reflects long-term investment in basic science and years of teamwork from many people at Cincinnati Children’s, NCATS, and the start-up company Kurome Therapeutics.
For the people who have found no successful treatment yet for their advanced acute myeloid leukemia (AML), joining a new clinical trial of the investigational drug KME-0584 might be the beginning of a life-changing journey.
If all goes well, those journeys could begin within months for up to 100 patients.
For Daniel Starczynowski, PhD, a cancer biologist and director of the new Advanced Leukemia Therapies and Research Center at Cincinnati Children’s, reaching this moment reflects 19 years of largely behind-the-scenes scientific labor. Not just his own, but the combined efforts of a small army of co-inventors, research collaborators, institutional leaders, philanthropists, investors and drug development experts.
“This is one of only a very few therapeutics to ever be developed at Cincinnati Children’s from the initial concept all the way to a clinical candidate with a filed IND (Investigational New Drug application) that’s open to begin clinical trials,” Starczynowski says. “I think this helps establish a blueprint of how drug discovery can be done at a place like this. It’s an encouraging message for leadership that their investments are moving forward and for the scientific community that sometimes the observations they make and the discoveries they publish can actually make their way to the clinic.”
What is KME-0584?
This is the research name created by the startup company Kurome Therapeutics for a small-molecule compound that inhibits the function of not just one, but two critical proteins associated with relapsed or refractory AML and a related condition known as high-risk myelodysplastic syndrome (HR-MDS).
After years of study, Starczynowski and colleagues determined that inhibiting both IRAK1 and IRAK4 kinases is necessary for controlling this rare but often fatal form of leukemia.
Every year, more than 20,000 people in the United States are diagnosed with AML and another 20,000 to 30,000 are diagnosed with MDS, according to the National Institutes of Health. Most cases occur in older adults. However, AML also affects about 600 children in the U.S. each year, making it the second most common blood cancer in children, after acute lymphoblastic leukemia (ALL).
The disease occurs when the bone marrow produces too many abnormal immature cells called myeloid blasts. These cells fail to mature into normal white blood cells. As they build up in the bone marrow and blood, they prevent other healthy blood cells from forming. This can result in life-threatening conditions including anemia, excessive bleeding and bruising, and hard-to-manage infections.
A variety of treatments already exist for AML. However, about 25% of patients do not achieve remission, and about half of those who do achieve remission experience a relapse. Very few treatments for HR-MDS are available.
These are the tough cases that the inventors of KME-0584 hope to address.
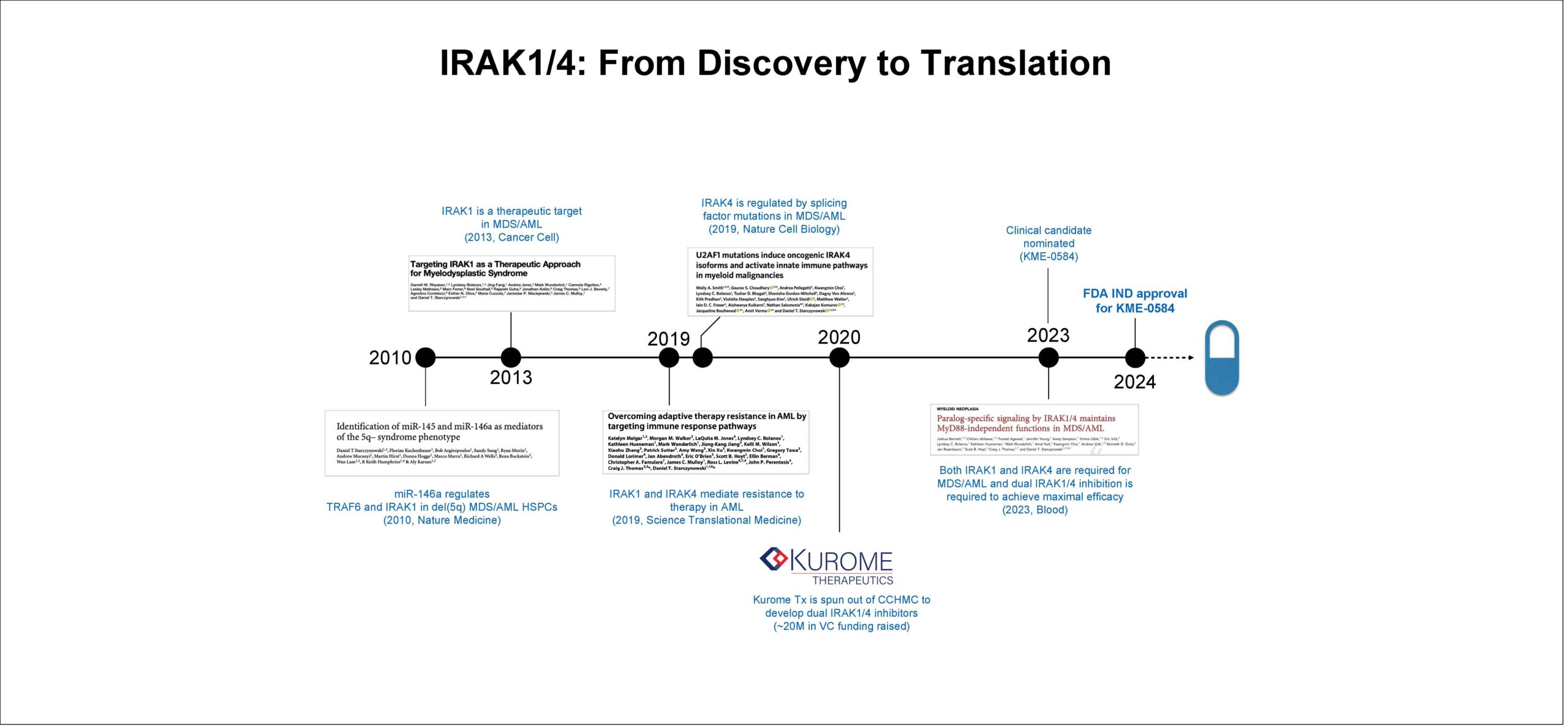
Scientific journey began two decades ago
The first clues that led to the new potential treatment emerged in late 2009, when Starczynowski published findings in Nature Medicine.
For the previous five years, Starczynowski, a native of Canada, had been working as a post-doctoral fellow at the University of British Columbia in Vancouver. There, he led work to characterize the function of a microRNA called miR-146a. This appeared to play a big role in a gene signaling pathway associated with bone marrow disorders that can lead to AML. The top targets of miR-146 were the genes TRAF6 and IRAK1, which normally function to regulate inflammatory signaling. However, in MDS and AML when miR-146 is deleted, TRAF6 and IRAK1 signaling become activated and contribute to the emergence of MDS and AML.
Starczynowski joined Cincinnati Children’s in 2010. He was recruited here by a cancer research team that included Yi Zheng, PhD; John Perentesis, MD; Stella Davies, MBBS, PhD; Jose Cancelas, MD, PhD; H. Leighton Grimes, PhD; and James Mulloy, PhD.
“I was impressed at the world-renowned expertise in experimental hematology and leukemia they had in the Cancer and Blood Diseases Institute. It was the ideal fit for the type of work I wanted to do as a fundamental cancer biologist,” Starczynowski says. “So for me this was the ideal supportive and collaborative environment to launch my career.”
Over the years since, Starczynowski has authored or co-authored more than 100 research papers. Along the way, he became a naturalized U.S. citizen in May 2022.
Much of his work at Cincinnati Children’s delved ever deeper into the role of aberrant inflammatory signaling, and specifically the dual functions played by the kinases IRAK1 and IRAK4, in MDS and AML.
“These two kinases are basically partners in crime,” Starczynowski says. “We had implicated both individually in these diseases, but the idea had always been that if you target one, it would effectively block signaling involving the other. In recent studies, we established that inhibiting both was required to disrupt the critical pathway in AML. In fact, we have recently published that there is functional compensation occurring when either one is blocked alone. This was never appreciated in the past.”
NCATS library kicks off a drug development chase
As the role of IRAK1 and IRAK4 became more clear, Starczynowski started working with Craig Thomas, PhD, a leading chemistry and drug development expert at the National Center for Advancing Translational Sciences (NCATS).
Established in 2011, NCATS is an arm of the NIH that receives federal funding to help researchers accelerate the translation of lab discoveries into new treatments. Thomas and Starczynowski combed through NCATS’ library of small molecule data and found a potential “hit.”
“Once they found a hit, we validated the molecule in our lab,” Starczynowski says. “Then they did some further chemistry optimization on the molecule and we arrived an optimized hit.”
Thomas, joined by lead chemist Scott Hoyt, PhD, and many others are credited as co-inventors of the drug now called KME-0584.
Just 1,600 more steps to go
For many scientific advances, reporting an optimized “hit” can be the final outcome of the work.
After years of experiments, the lab team confirms a connection between one or more genes and how their signals cause a normal bodily function to go sideways and cause disease. Some research teams delve a step further into the translational process by going on to report that an existing drug, or maybe an entirely new compound, is known to specifically affect that novel genetic signaling process.

Whether that hopeful–sounding compound can be used as a real drug that’s safe and effective in humans is largely left to others – primarily big pharmaceutical companies – to figure out.
Enter Kurome Therapeutics.
One challenge that has long bedeviled drug development for uncommon and rare diseases has been that big, profit-focused corporations may not see enough market potential in an interesting idea to justify further investment. This reality has prompted non-profit and government research institutions around the world to do more of the pre-clinical, ramp-up work themselves.
This is the path that Starczynowski’s potential AML treatment concept needed to follow.
In 2020, CincyTech, a seed investment and business incubating organization, announced the formation of Kurome Therapeutics to serve as the preclinical stage drug development company. Kurome licensed the technology from Cincinnati Children’s, named Jan Rosenbaum, PhD, as president and chief executive officer, and Starczynowski as chairman of the company’s scientific advisory board.
In 2021, the company announced raising $15 million in a “Series A” early-stage funding round. Those funds helped Kurome take that initial small-molecule “hit” much further.
“We generated more than 1,600 new derivatives to get to our clinical candidate,” Starczynowski says. “After finding that initial optimized hit, it was a huge lift to get to the actual clinical candidate. The collaborative effort between Kurome, NCATS, and Cincinnati Children’s led to an enormous amount of medicinal chemistry, optimization, and validation and as a result we have a real drug on hand that has exquisite properties.”
The research team synthesized molecule after molecule, running a series of tiered assays to learn about their basic properties, then selecting promising versions for even more analysis.
“The FDA expects to know how the compound circulates through the body, how it gets processed, how it binds to our targets, and for how long,” Starczynowski says. “There’s an enormous amount of information we have to know about how the drug is going to work and what are its potential toxicities.”
Doing all those tests – with little to no fanfare, long after publishing the key discoveries in respected medical journals – is required for obtaining an IND, he says.
A grand slam in a game without end
If getting published in a top journal feels like a home run for science, pushing that discovery to obtaining an IND is more like a grand slam.
But the game is far from over. The first doses in the clinical trial have yet to be administered. The outcomes for those patients remain to be seen. Whether this, and perhaps other clinical trials to follow, will result in an FDA-approved medication to combat MDS and AML will still take several years to determine. For a small biotech company like Kurome, partnering with larger companies or pursuing acquisition opportunities may be essential for advancing to clinical trials.
“I think everyone is aware that this entire effort can turn on a dime, at any moment. Nevertheless, this is a really important milestone for Cincinnati Children’s,” Starczynowski says. “There are not many examples in the history of Cincinnati Children’s where we’ve generated a new medication from the start. This might be one of those successes. We certainly hope it will be.”
Don’t Miss a Post:
- Subscribe to the Research Horizons Newsletter
- Follow Cincinnati Children’s Research Foundation on X: @cincyresearch
Research By

The Starczynowski Laboratory is interested in the molecular, cellular, and genetic basis of hematologic malignancies, with a specific focus on myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML).